World-first malaria vaccine rollout for children: What to know
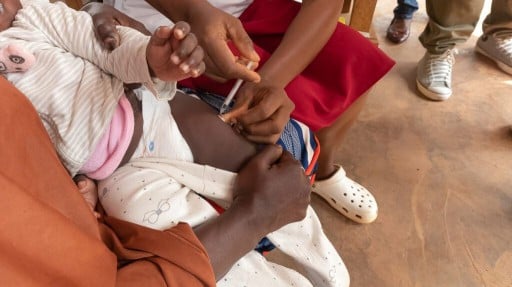
For the first time, an effective new malaria vaccine — RTS,S/AS01 — has rolled out in Cameroon, Africa.
RTS,S/AS01 has shown modest efficacy against symptomatic malaria in clinical trials.
The GSK-developed vaccine, also known as Mosquirix, is one of two malaria vaccines to receive approval from the World Health Organization (WHO).
WHO has also recommended the R21/Matrix-M vaccine, which shows 75% effectiveness against symptomatic malaria over 24 months.
An effective new malaria vaccine was rolled out for the first time in Cameroon, Africa, on January 22.
Global health officials say the immunization campaign for the RTS,S vaccine (Mosquirix) marks a historic moment in the fight against this mosquito-borne disease, one of the most life threatening infections in the world.
The vaccine drive in Cameroon is the first official public launch of the Mosquirix vaccine outside of clinical trials, which have shown a 13% reduction in all-cause mortality, including malaria-related deaths, in children who were eligible to receive it.
At least 20 African countries have made plans for malaria vaccine rollouts as cases and deaths related to the disease continue to climb. The majority of malaria cases occur on the continent, with an estimatedamong children each year.
But malaria is both preventable and curable, especially with the help of the new vaccine.
“We are not just witnessing, but actively participating in a transformative chapter in African public health history,” said Mohammed Abdulaziz, the head of disease control and prevention at Africa CDC, at a recent press conference.
“For a long time, we have been waiting for a day like this. It brings more than just hope. It brings a reduction in the mortality and morbidity associated with malaria,” Abdulaziz added.
Mosquirix ‘moderately effective’ against malaria
The rollout of a new malaria vaccine arrives as mosquitos have become increasingly resistant to insecticides and the Plasmodium parasite that causes malaria has become resistant to anti-malarial drugs.
However, the development of effective vaccines against malaria has been met with challenges due to the complexity of Plasmodium parasites. Widespread administration of effective vaccines in hard-hit areas has been slow as clinical trials have struggled to find sufficient evidence on malaria vaccine effectiveness.
, or Mosquirix, is one of two malaria vaccines to beby the World Health Organization (WHO) for the prevention of malaria in children. It was first developed in 1987, is manufactured by GSK, and has been studied extensively. It received itsin 2021.
The RTS,S/AS01 vaccine is a recombinant protein-based vaccine targeting the Plasmodium parasite before it invades and infects red blood cells.
Past studies conducted in Sub-Saharan Africa have shown that Mosquirix has only modest efficacy.
Ademonstrated that four doses of RTS,S/AS01 were 36% effective in children ages 5–17 months over a 48-month follow-up period.
notes a pilot implementation of the RTS,S/AS01 vaccine in Ghana, Kenya, and Malawi, is associated with a 30% reduction in hospital admissions for severe malaria.
By comparison, ashows another malaria vaccine, R21/Matrix-M, was 75% effective against first and multiple occurrences of malaria after three doses over a 24-month follow-up period. This vaccine wasin 2023.
The Mosquirix rollout in Cameroon now signals an opportunity to test this vaccine’s effectiveness on a larger scale in real-world settings.
“The burden of malaria is so high in Africa, with over 95% of fatal cases occurring in that continent, that even a moderately effective vaccine would have a huge impact on the epidemic,” Dr. Monica Gandhi, MPH, an infectious diseases specialist with the University of California, San Francisco, noted to Medical News Today.
“The two WHO-approved vaccines for malaria reduce cases by about 50% after one shot among children 5 months and older and by 75% if given seasonally. The roll-out in Africa (here in 42 districts in Cameroon) is a historic achievement that should curb childhood mortality from this preventable infectious disease. The main disadvantage of this vaccine is that is likely to have to be given annually for full effect.”
— Dr. Monica Gandhi, infectious disease specialist
What to know about malaria
In 2022, as many asdeaths were caused by malaria worldwide.
Around 95% of malaria deaths occur in Sub-Saharan African countries, and as much as 80% of those deaths occur in children under 5 years old.
Malaria is caused by unicellular parasites from the genus, which are transmitted through mosquito bites. Five species of Plasmodium may cause malaria in humans, but Plasmodium falciparum is the main cause of severe illness and death.
When a human is bitten by female mosquitoes infected with Plasmodium, the parasites enter the bloodstream and eventually infect the liver. Here, they multiply for 7–10 days, rendering the host asymptomatic. Symptoms occur once the parasites reenter the bloodstream and infect red blood cells, causing them to burst.
In addition to the new malaria vaccine, other preventive measures to protect against malaria include insecticides and antimalarial drug treatments.